Farmers with Official Tuberculosis Free status should be allowed to use APHA approved but currently un-validated tests for bovine TB, veterinary experts say.
PBD Biotech, developers of rapid bTB blood test Actiphage, have welcomed the recommendation as it 'empowers' farmers and their vets to improve biosecurity.
The British Veterinary Association (BVA) report includes research showing that farmers feel powerless in the bTB battle and the current blame culture is creating ‘fatigue’ that is hindering innovation.
It says the impact on the wellbeing of farmers and vets underlines the need for a 'new approach' to bTB management that involves all stakeholders.
The BVA policy states: “We support the use of non-statutory testing, particularly for farms that are part of a wider programme of earned recognition.”
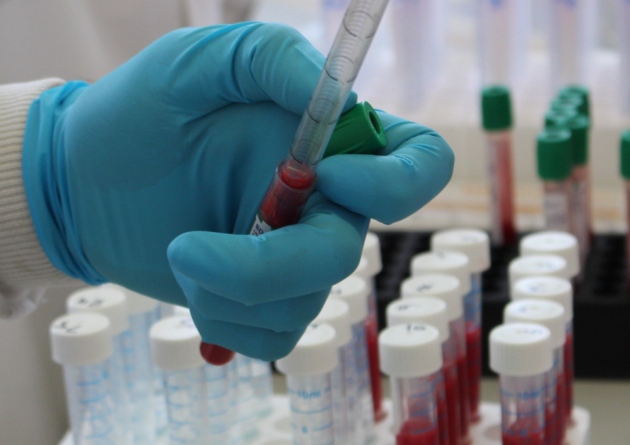
Actiphage has been approved by the APHA for exceptional private use in areas of chronic TB infection but once the disease is under control the permission is removed, stifling its use by farmers and their vets.
Mark Hammond, CEO of PBD Biotechnology said: “We know of farmers and private vets that would like to use Actiphage to accelerate the removal of infected animals and reduce the risk of within-herd transmission.
"Offering this flexibility would empower farmers to feel ownership of the disease management.”
Actiphage is the only test that directly identifies the presence of live Mycobacterium bovis, the pathogen that causes bTB, from a sample of blood.
It offers potential as a DIVA test as it can distinguish between live and dead mycobacteria.
Such a test is pre-requisite for a vaccine, and was identified as a priority for government investment by the BVA report.
Mr Hammond said: “The success of any system of bTB controls in cattle is dependent on the ability to detect the presence of infection, at herd level but also in individual live animals.
“The current skin test SICCT uses an immune response. While it is a good herd level test it only has 50-80% sensitivity so will miss individuals, leaving a reservoir of disease in the herd.
"A combination of approaches is required and encouraging trials of new diagnostics will increase the armoury available to farmers.”
The BVA policy report also recommends evaluation of bTB diagnostics for testing potential wild reservoirs of disease as well as non-bovid farmed animals.
Actiphage itself has been shown to be effective in badgers, deer and in total twenty other species of animal.
The speed of the diagnostic could also allow it to be used to test herd animals before and after movement, giving the opportunity to limit potential spread of the disease via a testing and tracing programme.
What recommendations have BVA made?
• Use of tests - The relevant authority should permit the exceptional private use of non-approved tests for bTB on cattle under certain conditions, with reactors statutorily notified and the herd remaining OTFW until the usual two tests.
• Additional testing - Government should build on the success of the rollout of IFN? and encourage research and trials to assess the potential for additional tests or combinations of tests
• Vaccine - Government should continue to prioritise the development of a cattle vaccine and DIVA test (a test that can differentiate between infected and vaccinated cattle).
• TB in other species - Government should seek to evaluate and validate existing bTB tests for susceptible non-bovine farmed species.
• Sharing information with vet - Where possible the results from bTB testing should be automatically shared with a farmer’s private vet, to allow a swift, coordinated response between all parties.
• Movement - Government should thoroughly evaluate the effect of the introduction of pre- and post-movement test requirements.
